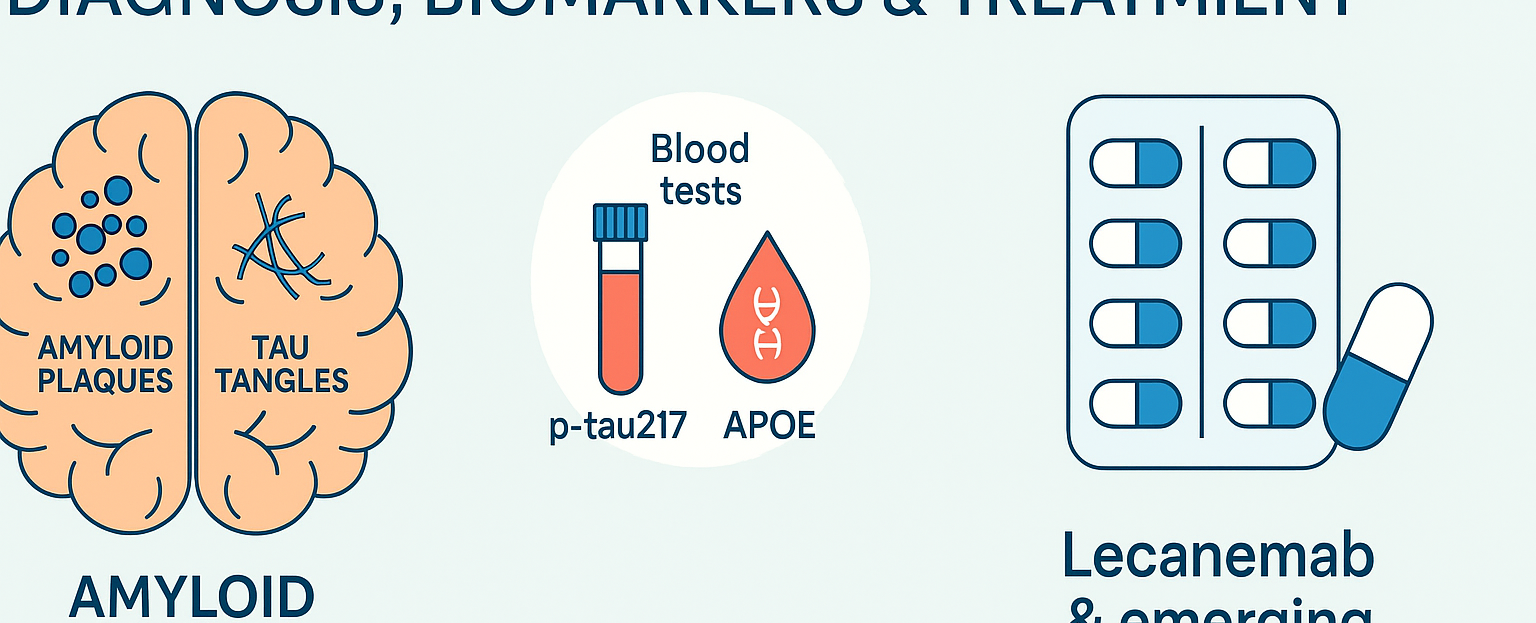
What is Alzheimer’s disease?
Alzheimer’s disease (AD) is the most common cause of dementia, a progressive brain disorder that slowly destroys memory, thinking skills and eventually the ability to carry out the simplest tasks. nhs.uk+3National Institute on Aging+3alzheimers.gov+3
The hallmark features include build-up of amyloid-β (“plaques”) and tau protein (“tangles”) in the brain, leading to nerve-cell death and brain tissue loss. Medscape+1
Symptoms typically begin with short-term memory loss or difficulty finding words, then progress through mild, moderate to severe dementia. Alzheimer’s Association+1
Key facts
-
It is progressive and currently irreversible. CDC+1
-
It is the most common cause of dementia in the UK and globally. nhs.uk+1
-
Risk factors include older age, genetics (especially the APOE ε4 allele), cardiovascular disease, diabetes, lifestyle. Wikipedia+1
Emerging blood-tests & biomarkers: p-tau217 and APOE
Until recently, diagnosis of Alzheimer’s disease relied on cognitive testing, brain scans (PET), cerebrospinal fluid (CSF) analysis and clinical symptoms. But advances are now introducing less invasive biomarkers.
Plasma p-tau217
Research shows that phosphorylated tau at the 217-amino-acid site (p-tau217) in blood correlates strongly with Alzheimer’s pathology (both amyloid and tau). JAMA Network
In the UK a large trial has just launched to evaluate a simple £100 blood-test measuring p-tau217, aiming to transform Alzheimer’s diagnosis in the NHS. Alzheimer's Society+1
This means quicker, more accessible diagnosis – which is key given the advent of disease-modifying therapies. University College London
Apolipoprotein E (APOE) genotyping
The APOE gene, and specifically the ε4 variant, is a major genetic risk factor for Alzheimer’s disease (increasing both risk and lowering age of onset). Wikipedia+1
While not a diagnostic test by itself, APOE genotyping is increasingly used in clinical trials and is recommended prior to certain therapies (see below) to stratify risk (for example for ARIA risk). PubMed+1
Practical implications for specialists
-
Integrate biomarker testing early in suspected cases of MCI (mild cognitive impairment) due to Alzheimer’s or early Alzheimer’s dementia.
-
Consider p-tau217 in blood as a screening tool, followed by confirmatory imaging/CSF if positive.
-
When planning advanced therapies, APOE genotype may guide safety counselling and monitoring.
-
Use these tests to enable earlier interventions (lifestyle + pharmacologic) when cognition is still preserved.
Management and latest drug therapies
Standard/Supportive management
Even before disease-modifying therapies, optimal management of Alzheimer’s disease includes:
-
Cognitive stimulation, structured activities, brain-health lifestyle (exercise, diet, sleep, social engagement).
-
Management of cardiovascular risk factors (hypertension, diabetes, hyperlipidaemia) which may slow progression.
-
Symptomatic pharmacotherapy (e.g., cholinesterase inhibitors, memantine) although benefit is modest. Wikipedia+1
-
Multidisciplinary care: specialist input (neurology/gerontology), memory clinics, carer support planning, advanced care planning.
Disease-modifying therapies – focus on Lecanemab
Lecanemab (brand name LEQEMBI®) is a monoclonal antibody targeting amyloid-β protofibrils. It has been approved for early Alzheimer’s disease (mild cognitive impairment due to AD or mild AD dementia) in patients with confirmed brain amyloid pathology. PMC+2Alzheimer’s Association+2
Clinical trial results show that lecanemab reduced amyloid burden and resulted in a modest but statistically significant slowing of cognitive and functional decline. New England Journal of Medicine+1
Key prescribing/monitoring points
-
Confirm amyloid pathology (e.g., PET/CSF) prior to initiation. PMC+1
-
Baseline MRI is required to exclude microhaemorrhages, siderosis etc. ASPE+1
-
ApoE genotype assessment recommended (especially for APOE ε4 carriers) to estimate risk of amyloid-related imaging abnormalities (ARIA). PubMed+1
-
Administration: 10 mg/kg IV twice monthly initially; after ~18 months maintenance dosing may shift to once every 4 weeks. leqembihcp.com
-
Monitoring for ARIA (brain oedema or micro-haemorrhage) is essential. Leqembi+1